Chromatography is the separation of a mixture of compounds (solutes) into separate components. By separating the sample into individual components, it is easier to identify (qualitative) and measure the amount (quantitate) of the various sample components. There are numerous chromatographic techniques and corresponding instruments. Gas chromatography (GC) is one of these techniques. It is estimated that 10-20% of the known compounds can be analysed by GC. To be suitable for GC analysis, a compound must have sufficient volatility and thermal stability. If all or some of a compound’s molecules are in the gas or vapor phase at 400-450°C or below, and they do not decompose at these temperatures, the compound can probably be analysed by GC.
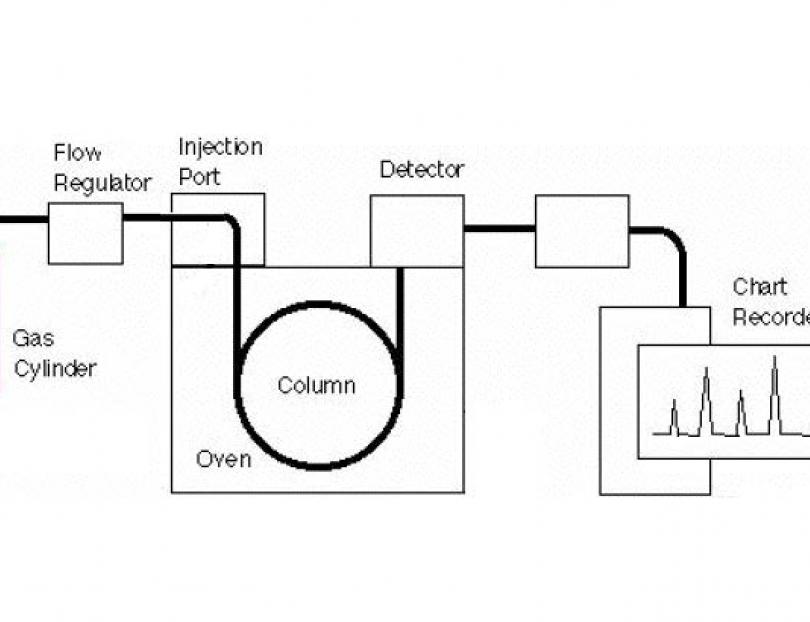
The main parts of a basic GC system are shown in the above diagram. One or more high purity gases are supplied to the GC. One of the gases (called the carrier gas) flows into the injector, through the column and then into the detector. A sample is introduced into the injector usually with a syringe or an exterior sampling device. The injector is usually heated to 150-250°C which causes the volatile sample solutes to vaporize. The vaporized solutes are transported into the column by the carrier gas. The column is maintained in a temperature controlled oven. The solutes travel through the column at a rate primarily determined by their physical properties, and the temperature and composition of the column. The various solutes travel through the column at different rates. The fastest moving solute exits (elutes) the column first then is followed by the remaining solutes in corresponding order. As each solute elutes from the column, it enters the heated detector. An electronic signal is generated upon interaction of the solute with the detector. The size of the signal is recorded by a data system and is plotted against elapsed time to produce a chromatogram.
The ideal chromatogram has closely spaced peaks with no overlap of the peaks. Any peaks that overlap are called co-eluting. The time and size of a peak are important in that they are used to identify and measure the amount of the compound in the sample. The size of the resulting peak corresponds to the amount of the compound in the sample. A larger peak is obtained as the concentration of the corresponding compound increases. If the column and all of operating conditions are kept the same, a given compound always travels through the column at the same rate. Thus, a compound can be identified by the time required for it to travel through the column (called the retention time). The identity of a compound cannot be determined solely by its retention time. A known amount of an authentic, pure sample of the compound has to be analysed and its retention time and peak size determined. This value can be compared to the results from an unknown sample to determine whether the target compound is present (by comparing retention times) and its amount (by comparing peak sizes). If any of the peaks overlap, accurate measurement of these peaks is not possible. If two peaks have the same retention time, accurate identification is not possible. Thus, it is desirable to have no peak overlap or co-elution.






