February 2021
Tingwen Zhao - School of Chemistry
We have lots of people in the EMU who are acquiring lovely images and great data. If you have an image that you think is good, please submit it to the EMU image of the season competition*. Every three months the EMU will award Three (3) prizes for the image of the season: First prize is a $100 gift voucher from the UNSW bookstore, and Two runner-ups will receive a $50 gift voucher from the UNSW bookstore. All submissions are voted on by EMU staff. Your winning entry will be displayed in the EMU and put on our website.
Submit your entry using the EMU Image of the Season form
*Only one submission per person per season.
*Each person may only win one prize each calendar year.
Tingwen Zhao - School of Chemistry
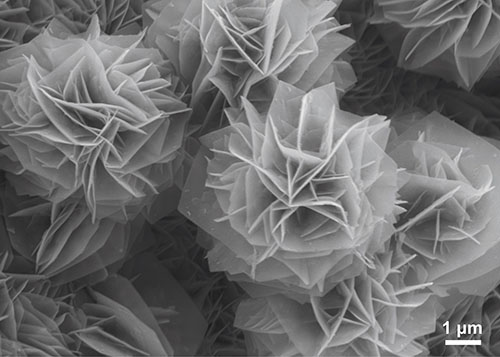
This image shows the in-situ growth of porous flower-like nickel phosphides on conductive nickel foam as an electrocatalyst for water splitting. The three-dimensional flower-like morphology is formed with numerous two-dimensional ultrathin nanosheets, thereby significantly enlarging the porosity and electrochemical surface area.
Image by: Tingwen Zhao
Supervisor: Prof. Chuan Zhao
Microscope/Technique: JEOL 7001f HRSEM
Yong Zhao - School of Chemistry
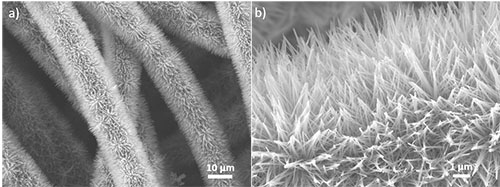
Zinc oxide nanowires with a diameter of tens of nanometers and a length of several micrometers are neatly and radially grown on the surfaces of woven carbon fibres. The tips of these nanowires aggregate together forming ZnO nanowire bundles.
Image by: Yong Zhao
Supervisor: Prof. Chuan Zhao
Microscope/Technique: JEOL 7001f HRSEM
Derrick Lau - School of Medical Sciences, EMBL Node for Single Molecule Science

Alpha synuclein is a protein that is mainly found in neuronal cells. While its function is not clear, it has been identified as a potential biomarker in helping diagnosing patients with synucleinopathy disorders. Aggregation of alpha synuclein is associated with various neurodegenerative disorders such as Parkinson disease but the formation of these aggregate remains poorly understood. Here, we purified recombinant alpha synuclein and these purified proteins form long fibrils with an average diameter of 16-17 nm. These so-called preformed-fibrils recapitulate certain characteristics of alpha synuclein aggregates and can be sonicated to produce short seeds for diagnostic assays looking at identifying an individual with neurodegenerative disorders.
Image by: Derrick Lau
Supervisors: Dr Yann Gambin, Dr Emma Sierecki and A/Prof Antony Cooper
Microscope/Technique: FEI Tecnai G2 20, negative staining (2% w/v uranyl acetate)
Lucy Fillbrook - School of Chemistry
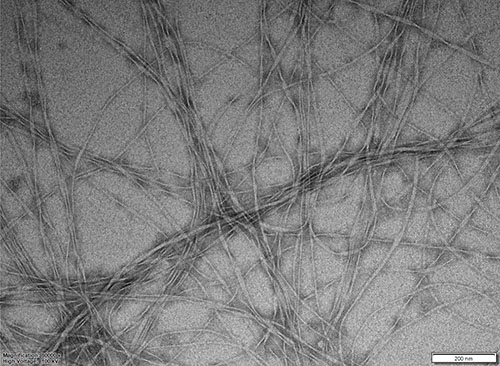
TEM image of arylazopyrazole-based hydrogel in water. The long, thin fibres wind and intertwine leading to the formation of a stiff gel.
Image by: Lucy Fillbrook
Supervisors: Dr Jonathon Beves
Microscope/Technique: JEOL JEM1400 TEM
Yingzhu Zhou - School of Chemical Engineering, Australian Centre for Nanomedicine
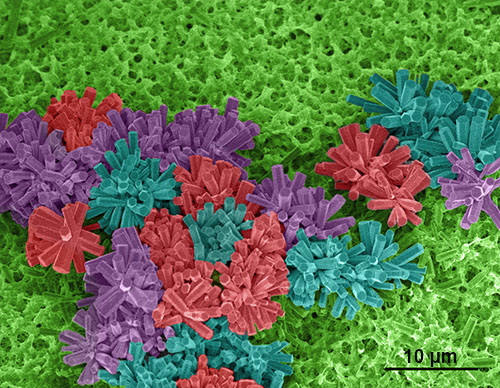
SEM image of flower-shaped zinc-oxide (ZnO) rods grown on titanium wire. The image was coloured to create a cluster of "flowers" on the "grass" (wire). ZnO coatings act as a catalyst for the generation of nitric oxide, a signalling molecule involved in several cardiovascular and neurological processes. ZnO synthesis by Dr. Fabio Lisi.
Image by: Yingzhu Zhou
Supervisor: Dr Rona Chandrawati
Microscope/Technique: FEI NanoSEM 230
Yokasundery Muniandy - School of Mechanical and Manufacturing Engineering
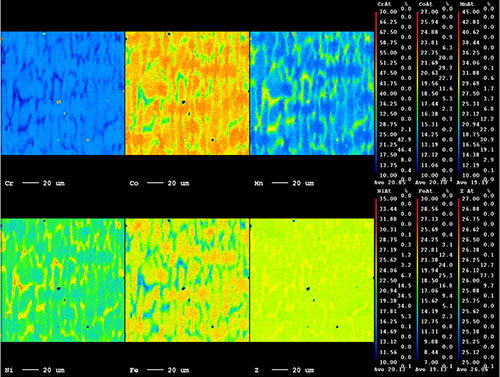
5-component high-entropy alloy (HEA) consisting of Cr-Mn-Fe-Co-Ni assume to be single-phase. The quantified average composition reflect equaitomic, but the microstructure shows rather a complex and dynamic element profile (WDS quantified map).
Image by: Yokasundery Muniandy
Supervisor: Dr. Bernd Gludovatz
Microscope/Technique: JEOL JXA-8500F EPMA Hyperprobe/Quantitative WDS
Ali Alinezhad Chamazketi - School of Chemistry
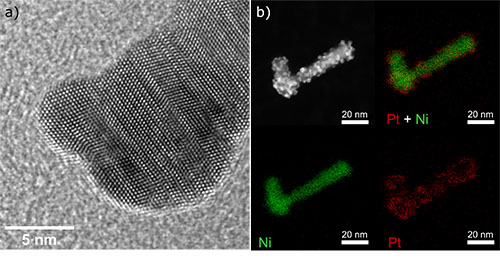
a) Atomic resolution HRTEM image of a branched nickel nanoparticle decorated with strained platinum islands. the amount of strain was tuned by changing the size of the Pt islands to achieve improved HER activity.
b) STEM-EDX mapping of a typical branched Ni nanoparticle decorated with Pt islands.
Image by: Ali Alinezhad Chamazketi
Supervisor: Prof. Richard Tilley
Microscope/Technique: JEOL f200 TEM, HRTEM, EDX
Electron Microscope Unit:
Basement
June Griffith Building (Chemical Sciences - F10)
Kensington UNSW Sydney
NSW 2033
Tel: +61 (2) 9385 4425
Fax: +61 (2) 9385 6400
Email: EMUAdmin@unsw.edu.au

Authorised by the Executive Director of Mark Wainwright Analytical Centre, UNSW CRICOS Provider Code 00098G, ABN 57 195 873 179
Mark Wainwright Analytical Centre, UNSW, Sydney, NSW, 2052, Australia | Email: analytical@unsw.edu.au
