The Outreach group POM committee is pleased to announce the ‘Paper of the Month’ for September 2016 is a study contributed to by Ling Zhong and Mark Raftery from BMSF, in conjunction with:
Sibasish Dolai, Keith C.S. Sia, Alissa K. Robbins, Sue L. Heatley,Tiffaney L. Vincent, Falko Hochgräfe, Rosemary Sutton, Raushan T. Kurmasheva, Tamas Revesz, Deborah L. White, Peter J. Houghton, Malcolm A. Smith, David T. Teachey , Roger J. Daly, Mark J. Raftery, and Richard B. Lock
Quantitative phosphotyrosine profiling of patient-derived xenografts identifies therapeutic targets in pediatric leukemia.
Published in: Cancer Research. 2016, 76, 9, 2766-2778
(impact factor 9.3)
The study highlights the application and potential of phosphotyrosine profiling using mass spectrometry for identifying clinically relevant kinase targets in leukaemia.
Activating mutations in tyrosine kinases (TK) drive paediatric high-risk acute lymphoblastic leukemia (ALL) and confer resistance to standard chemotherapy. Therefore, there is urgent need to characterize dysregulated TK signalling axes in patients with ALL and identify actionable kinase targets for the development of therapeutic strategies. Here, we present the first study to quantitatively profile TK activity in xenografted patient biopsies of high-risk pediatric ALL. We quantified 1394 class I phosphorylation sites in 16 ALL xenografts. Moreover, hierarchical clustering of phosphotyrosine sites could accurately classify these leukemias into either B- or T- cell lineages with the high-risk early T-cell precursor (ETP) and Ph-like ALL clustering as a distinct group. Furthermore, we validated this approach by using specific kinase pathway inhibitors to perturb ABL1, FLT3, and JAK TK signaling in four xenografted patient samples. By quantitatively assessing the tyrosine phosphorylation status of activated kinases in xenograft models of ALL, we were able to identify and validate clinically relevant targets.
To read more, follow this link: http://www.ncbi.nlm.nih.gov/pubmed/26960974
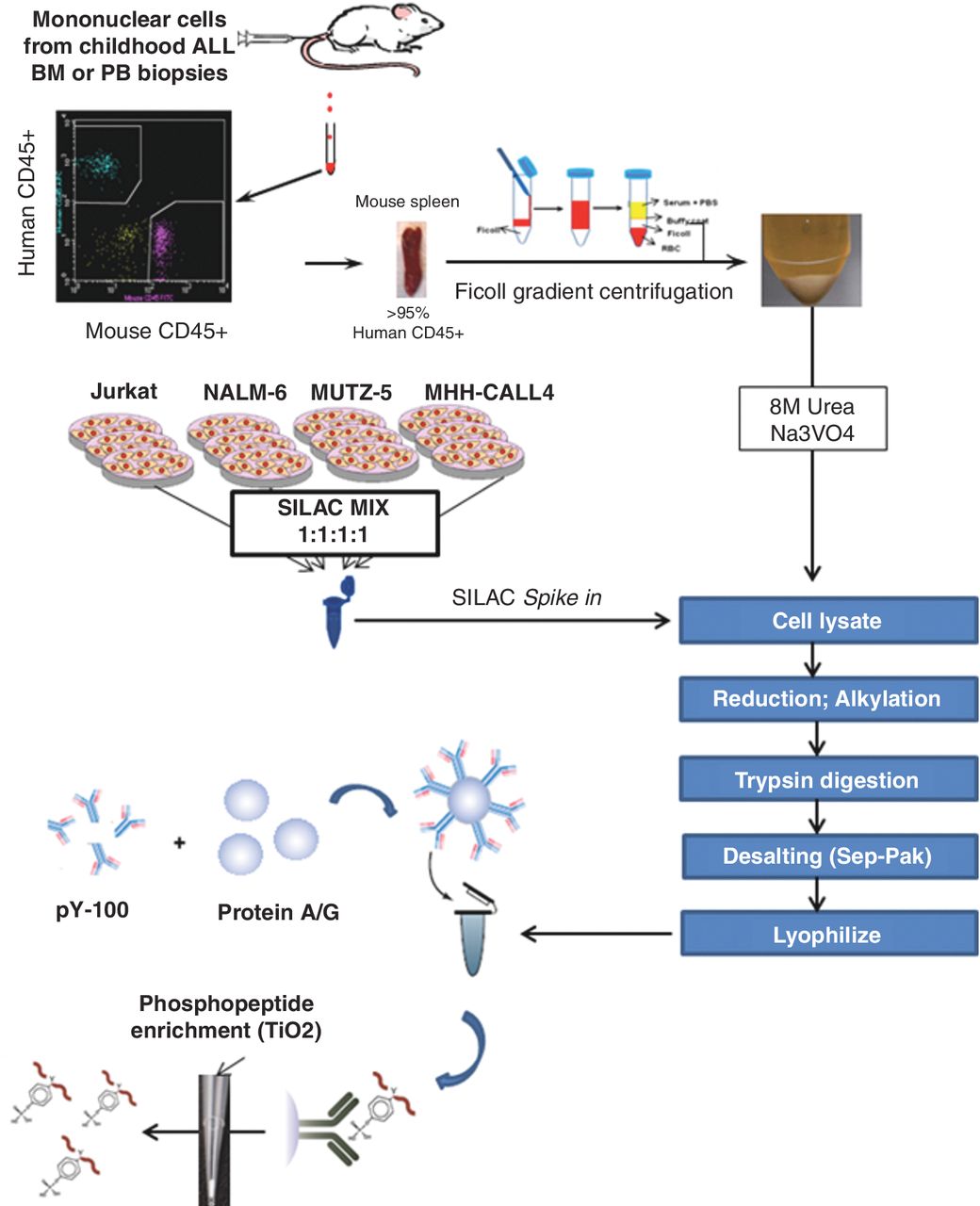
Schematic representation of “spike-in” SILAC quantitative phosphotyrosine profiling. The purified peptides are enriched for tyrosine phosphopeptides using pY-100 antibody-bound protein A/G beads. Eluted phosphopeptides undergo mass spectrometric analysis.






